
Sarepta Shares Soar After FDA Reverses Pause For Controversial Gene Therapy Elevidys
Shares of Sarepta Therapeutics erupted in premarket trading after the Food and Drug Administration (FDA) lifted a voluntary pause on its gene therapy Elevidys, limited to patients who can still walk. The decision follows multiple deaths linked to the drug, which is used to treat Duchenne muscular dystrophy.
„Sarepta will resume shipping ELEVIDYS to sites of care for treatment of ambulatory patients with Duchenne imminently,” the pharmaceutical company stated in a press release on its website. This comes ten days after the FDA requested Sarepta to halt all shipments of the drug after three deaths. Sarepta initially refused, then caved a few days later.
Sarepta CEO Doug Ingram wrote in a statement:
„Last week, at the suggestion of FDA, Sarepta made the difficult decision to pause shipments of ELEVIDYS to provide the FDA with an opportunity to complete a review of available safety information. We are very pleased that FDA chose to rapidly and comprehensively complete that review and to recommend that we remove our voluntary pause and resume shipment of ELEVIDYS for ambulatory patients. The FDA’s swift review evinces a commitment to the Duchenne population, a commitment shared by Sarepta.
„We look forward to working collaboratively with the FDA to complete the safety label update for ELEVIDYS and to discussing the approach to risk-mitigation for non-ambulatory patients, who remain on pause pending the outcome of those discussions.”
Initial reactions from Wall Street desks suggest that a partial overhang has been lifted for Sarepta, helping to stabilize the company’s financial outlook ahead of payments to a biotech partner and $1.2 billion in debt maturing in 2027.
Analyst commentary (courtesy of Bloomberg):
BMO (market perform, PT raised to $50 from $25)
-
The FDA’s decision suggests that fatality risk with Elevidys is in ambulatory patients and largely independent of non- ambulatory patient risk, says analyst Kostas Biliouris
-
Sarepta’s financial overhang is partially removed, although physicians and patients may remain hesitant about the treatment in the near term
Jefferies (buy, PT $35)
-
The news significantly improves Elevidys’ sales outlook in the near-term and creates better visibility around the firm’s cost structure, says analyst Andrew Tsai
-
Street will be relieved about the situation, suggesting meaningful upside potential to the stock
JPMorgan (raises to neutral from underweight, PT $24)
-
The FDA update came rather quickly after Sarepta voluntarily paused shipments only one week ago, and should provide confidence in the financial outlook for the company, says analyst Anupam Rama
-
But understanding the Elevidys launch curve following this unprecedented regulatory situation could take a couple quarters, and „we worry about headline risk”
Morgan Stanley (equal-weight, PT raised to $20 from $15)
-
The FDA’s faster-than-expected review and favorable recommendation for ambulatory patients are encouraging, acknowledging the high unmet need and potential benefits of Elevidys, says analyst Michael Ulz
-
Expects the firm to receive about $500m per year for the next two years from Elevidys, up from a previous forecast of no revenue for 2026 and 2027
Sarepta noted:
FDA’s review of the safety data in the ambulatory population included the case of an 8-year-old in Brazil whose death was deemed unlikely to be related to treatment with ELEVIDYS by the Brazilian health authorities. FDA’s investigation has concluded the death was unrelated to treatment with ELEVIDYS and confirmed that Sarepta can resume shipments.
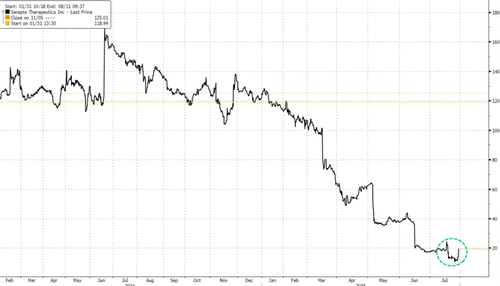
Shares of Sarepta jumped 38% in premarket trading, building on Monday’s 16% gain. Year to date, the stock is down 88%.
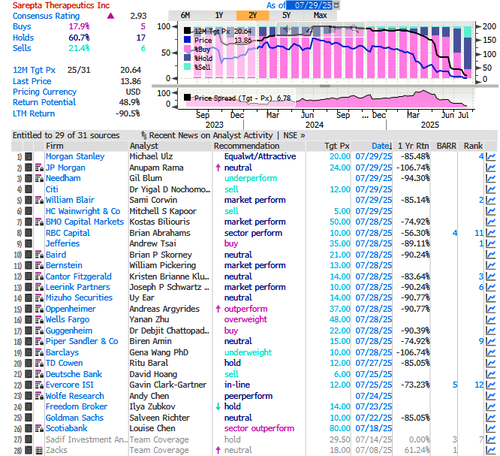
According to the latest Bloomberg data, there are six sell ratings, 17 holds, and five buys on the stock. The average 12-month price target among Wall Street analysts is $20.64.
Last week:
-
Sarepta Price Target Slashed To $0 At H.C. Wainwright As Stock Plunge Continues
-
Sarepta Plunges Again After Europe Rejects Elevidys
. . .
Tyler Durden
Tue, 07/29/2025 – 08:15








