
„Groundbreaking” FDA Shift Away From Animal Testing Sends Lab Shares Into Freefall
The U.S. Food and Drug Administration (FDA) announced a major policy shift late Thursday, signaling the replacement of traditional animal testing—particularly in monoclonal antibody development—with advanced computer modeling and artificial intelligence. The news sent shares of lab companies tumbling on the update, while shares in companies working on biotech AI models jumped.
„Today, the FDA is taking a groundbreaking step to advance public health by replacing animal testing in the development of monoclonal antibody therapies and other drugs with more effective, human-relevant methods,” the FDA wrote on X, twenty minutes before U.S. cash markets closed on Thursday.
Today, the FDA is taking a groundbreaking step to advance public health by replacing animal testing in the development of monoclonal antibody therapies and other drugs with more effective, human-relevant methods.https://t.co/m8vsOah6qx pic.twitter.com/Jkruz8PGV7
— U.S. FDA (@US_FDA) April 10, 2025
Here’s a summary of the FDA’s major shift in drug testing policy:
-
AI-based computer modeling to simulate drug behavior and toxicity.
-
Lab-grown human organoids and organ-on-a-chip systems to detect toxic effects more accurately than animal tests.
-
Use of real-world safety data from countries with comparable regulatory standards.
-
Regulatory incentives to encourage adoption of these alternatives, including potential streamlined reviews.
-
Immediate application to new drug applications (INDs), with a roadmap released and a public workshop planned.
„With this move, the FDA reaffirms its role as a global leader in modern regulatory science, setting new standards for the industry and encouraging the adoption of innovative, humane testing methods,” the FDA stated in a press release.
Here’s the response from Wall Street analysts (courtesy of Bloomberg):
Baird analyst Eric Coldwell
-
says „expect very slow change to the research animal and animal testing services market”
-
„Street got shock and awe news today, and players in this industry likely won’t fully recover when it comes to valuation,” Coldwell writes in a note
Jefferies analyst David Windley
-
says FDA’s Modernization 2.0 already stared phasing out animal testing, „but perhaps this is a stronger push with more prescribed plan”
-
„At the least with preclinical spending deprioritized, this is just another reason to pause.”
Barclays analyst Luke Sergott
-
says while taking out preclinical animal testing testing is „a good goal” — „questions on feasibility and implementation remain”
-
Charles River in the cross-hairs, however „this paradigm shift has downstream impacts to late-stage trials”
RBC Capital Markets analyst Brian Abrahams
-
says „these steps towards increased regulatory flexibility as net positive, especially given recent concerns about potential FDA dysfunction and potentially greater stringency”
-
Antibody innovators Regeneron Pharmaceuticals, Amgen, Viridian, BeiGene, Xencor and Ultragenyx Pharmaceutical would likely stand to benefit the most
The immediate consequence of the significant testing policy shift sent shares of Charles River, the leading provider of research animal models, plunging by a one-day record of 28%, reaching their lowest level since March 2020 by Thursday’s close.
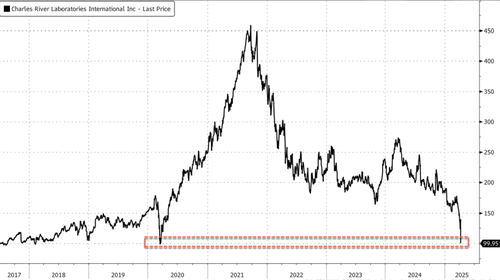
Peers also fell: LabCorp closed 5.2% lower, and Inotiv crashed 50%. Meanwhile, companies working on AI models surged in premarket trading, with Certara up 20%, Schrodinger up 15%, and Nuvation Bio up 3%.
Tyler Durden
Fri, 04/11/2025 – 13:05


















